

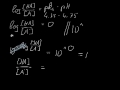




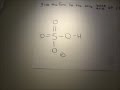



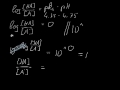





The conjugate base of an acid, any acid, is defined as the acid "LESS" a proton, H^+. The conjugate acid of a base, any base, is defined as the base "PLUS" a proton. Phosphoric acid, H_3PO_4, is the parent acid. If it loses a proton, H^+, we conserve both mass and charge, and H_2PO_4^- results. And what is the conjugate base of this beasty? The conjugate acid of H P O 4 2 − is H 2 P O 4 − H 2 P O 4 − → H + + H P O 4 2 − The base and its conjugate acid differ by one proton only. its an acid, H2PO4- is called dihydrogen phosphate ion. It is the conjugate base of Phosphoric Acid H3PO4 and the conjugate acid of monohydrogen phosphate ion HPO42 H3PO4. Within the Brønsted–Lowry acid-base theory (protonic), a conjugate acid is the acid member, HX, of a pair of two compounds that transform into each other by gain or loss of a proton (hydrogen ion). Conjugate Acid Of H2po4. Source(s): https://shrink.im/a0ffX. 0 0. Anonymous. 5 years ago. For the best answers, search on this site https://shorturl.im/8dmlP. congugate acid is H+ and conjugate base is PO4-0 0. Anonymous. 6 years ago. problematic subject. do a search on yahoo or google. just that can assist! 0 3. The conjugate base of H2PO4... chemistry. The conjugate base of H 2 Which of the following are conjugate acid-base pairs: This question has multiple correct options. View solution. Of the following complex ions, one is a Bronsted-lowry acid. That one is: View solution. Similarly, what is the conjugate base of the Bronsted Lowry acid hpo4 2 -? Hence an acid donates a proton to form it's corresponding conjugate base while a base accepts a proton to form it's corresponding conjugate acid. From Bronsted Lowry theory , we can conclude that the conjugate base of HPO4^-2 is PO4^-3. Simply so, what is the conjugate acid of hpo2 − 4? Table of acids and their conjugate bases Well, to get the conjugate base we REMOVE a proton. And to get the conjugate acid we ADD a proton… And so…[math]H_{2}PO_{4}^{-} + H^+ → H_{3}PO_{4}[/math] And thus phosphoric acid is the conjugate acid of [math]H_{2}PO_{4}^{-}[/math] and the conju... H3PO4 is a tribasic acid, thus ionising in three steps.The conjugate base is formed when an acid loses its proton. Thus, HPO42- is the conjugate base of H2PO4- (which is an acid in step II, but is the conjugate base of H3PO4 in step I) The conjugate system involves proton transfer. The conjugate acid of H2PO4- is H3PO4 or phosphoric acid with a added proton. If it is conjugate acid, add a proton then remove a negative charge. If it is conjugate base, remove a proton then add a negative charge.
[index] [172] [3640] [1546] [2124] [6576] [6289] [8701] [4026] [9056] [7380]
Here I am just answering one of my hw problems. I will try to post as much hw as I can This is for an OCHEM CLASS. All the answers provided are checked by th... Chemistry: Learn more at: http://www.pathwaystochemistry.com/chemistry-qa/videos/conjugate-acid-base-pairs/ Determine acid/base ratio of a buffer - Duration: 12:43. Peter Klappa 38,107 views. 12:43. Using the HH equation to calculate partial charges on amino acids - Duration: 5:26. ... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... In the Brønsted-Lowry definition of acids and bases, a conjugate acid-base pair consists of two substances that differ only by the presence of a proton (H⁺).... The content of this video is designed to accompany the 12th edition of "Chemistry The Central Science" by Brown, Lemay, Bursten, Murphy, and Woodward. The ti... Determine acid/base ratio of a buffer Learn everything about Conjugate Acids and Bases. We explain this with the real world example of vinegar.At Fuse School, teachers and animators come together...
Copyright © 2024 m.globalsportnews.site